Electrophilic Substitution Reaction Overview
An electrophile (an electron pair acceptor) replaces the functional group attached to a molecule in an electrophilic substitution process. In an electrophilic substitution, the displaced functional group is frequently a hydrogen atom. Electrophilic substitution, also known as electrophilic aromatic substitution reactions, occurs in many compounds containing benzene rings). The electrophilic aliphatic substitution process is a different type of electrophilic substitution reaction. Three stages make up the electrophilic substitution reaction: the synthesis of an electrophile, the creation of a carbocation that serves as an intermediary, and the extraction of a proton from the medium.
What is an Electrophilic Substitution Reaction?
A chemical reaction known as an electrophilic substitution reaction occurs when an electrophile replaces the functional group of a molecule. A hydrogen atom usually makes up the displaced functional group. The three steps that electrophilic substitution reactions commonly go through are as follows-
- Introduction of an electrophile.
- The creation of an intermediate carbocation.
- The process of taking a proton out of an intermediary.
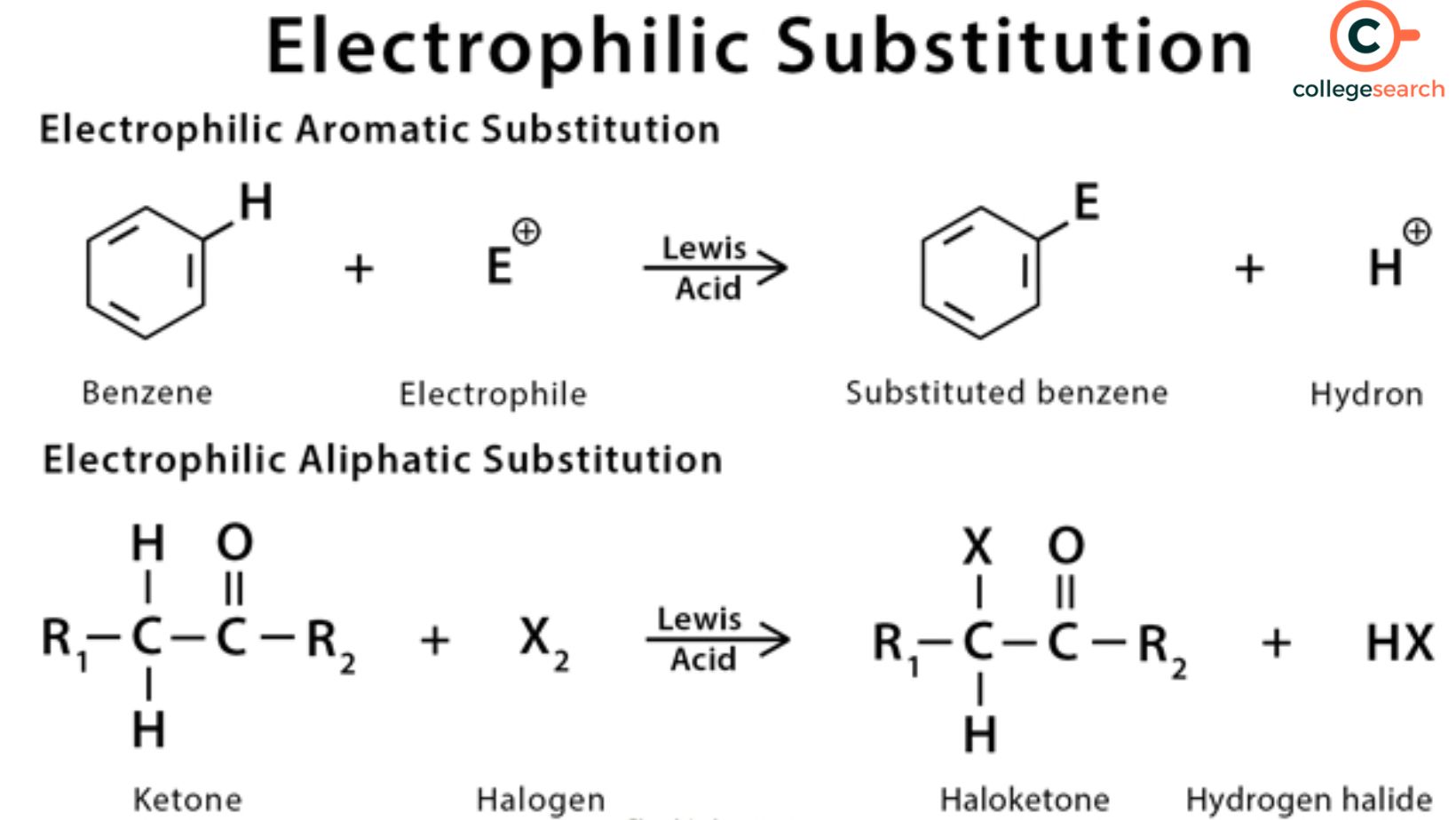
Example of Electrophilic Substitution Reaction
An atom that is connected to an aromatic ring is replaced with an electrophile in electrophilic aromatic substitution processes. Examples of these reactions include Friedel-Crafts reactions, aromatic nitrations, and aromatic sulphonations.

Importance of Electrophilic Substitution Reaction
The following are some of the reasons for the importance of substitution reactions-
- Electrophilic aromatic substitution is one of the most significant processes in synthetic organic chemistry.
- These reactions result in a major intermediate that can be employed as a precursor in the production of industrial, agrochemical, and pharmaceutical products.
- However, a lot of industrial processes for making these materials still rely on technology that was developed decades ago.
- Regioisomer mixtures are routinely produced by such procedures, and various additional techniques have been created recently to alter the processes' radiochemistry.
- Zeolites have a variety of uses, including helping reagents, acting as heterogeneous catalysts, entrapping byproducts, increasing product para-selectivity by shape-selectivity, and reducing watery work-ups.
- Halogenation, alkylation, para-regioselective nitration, acylation, and methanesulfonyl reactionpathways can all be advantageous for zeolites in moderate conditions.
- Additionally, they are frequently easy to filter out of the reaction mixture and then revitalize by heating, allowing for repeated usage with almost the same productivity and selectivity as samples.
Types of Electrophilic Substitution Reactions
Electrophilic aromatic substitution reactionsand electrophilic aliphatic substitution reactions are the two main forms of electrophilic substitution reactions that organic compounds experience. The example below demonstrates the electrophilic replacement of a hydrogen atom (from a benzene molecule) with a chlorine atom-
In this instance, the chlorine cation takes the place of a hydrogen atom in the benzene ring by acting as an electrophile. In this electrophilic substitution process, a proton and a chlorobenzene molecule are the end products. The following table gives details about the types of electrophilic substitution reactions-
|
Electrophilic Aromatic Substitution Reaction |
Electrophilic Aliphatic Substitution Reaction |
|
An atom that is connected to an aromatic ring is replaced with an electrophile in electrophilic aromatic substitution processes. |
It is significant to highlight that in electrophilic aromatic replacements, the aromaticity of the aromatic compound is maintained. |
|
Examples of these reactions include Friedel-Crafts reactions, aromatic nitrations, and aromatic sulphonations. |
As a result, using aromatic rings and iodine, bromine, or chlorine, these processes can be employed to produce aryl halides. |
An electrophile replaces the functional group (often hydrogen) in an aliphatic molecule in electrophilic aliphatic substitution processes. The following five categories can be used to group these reactions-
- Diazonium coupling (aliphatic)
- Halogenation of ketones
- Insertion of a carbene into a carbon-hydrogen bond
- Keto-Enol tautomerism
- Nitrosation
If the electrophilic assault is 180 degrees away from the leaving group (attack from behind), these electrophilic substitution reactions may lead to a configuration inversion.
Mechanism of Electrophilic Substitution Reaction
Three stages make up the electrophilic substitution reaction process-
Stage 1: Electrophile Generation
When an aromatic ring is chlorinated, alkylated, or acylated, an electrophile is produced. Anhydrous aluminum chloride is a particularly helpful Lewis acid in this process. The resultant electrophiles are Cl+, R+, and RC+O, respectively, as illustrated below (when anhydrous aluminum chloride and the attacking reagent are combined).
Stage 2: Carbocation Formation
By attacking the aromatic ring, the electrophile creates an arenium ion or a sigma complex. This arenium ion has an sp3 hybridized carbon. A resonance structure provides stability for this arenium ion. Sigma complexes or arenium ions lose their aromatic properties because electron delocalization ends at the sp3 hybridized carbon.
Stage 3: Proton Removal
The [AlCl4]- attacks the sigma complex, which then releases a proton from the sp3 hybridized carbon to reestablish the aromatic property. The following describes the reaction that happens when a proton is taken out of the Sigma complex-
As a result, the hydrogen atom in the benzene ring is changed by the electrophile. Due to the concept's widespread application in other chemical processes, the electrophilic substitution reaction is a crucial one in organic chemistry.

h2> Electrophilic Substitution Reaction of Anilines
A species-seeking electron is an electrophile. Thus, when one electrophile replaces another electrophile in an organic molecule, an electrophilic substitution process takes place. Common electrophilic reactions for anilines include halogenation, nitration, and sulfuration. Aniline has a functional group (-NH2) that donates electrons, making the electrophilic substitution reaction extremely active. Due to its several resonant topologies, the benzene ring has more electrons or negative charges in the ortho- and para-positions than in the meta-position. As a result, in the electrophilic substitution process, anilines are o- and p-directive.
Conclusion
A hydrogen atom is frequently the shifted functional group in an "electrophilic substitution." Numerous benzene-containing abstract compounds go through electrophilic substitution, also known as electrophilic aromatic substitution reactions. The "electrophilic aliphatic substitution reaction" is another type of electrophilic substitution reaction. Three phases make up the electrophilic substitution procedure: making an electrophile, creating a covalent bond that serves as an intermediate, and removing a proton from the medium.
